

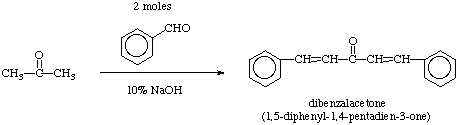
When an enolate forms from an aldehyde, the enolate will normally react with unreacted aldehyde to undergo the "aldol addition" or "aldol condensation" reaction. Since ketones are less reactive toward nucleophilic addition, the enolate formed from a ketone can be used to react with an aldehyde, a modification called the Claisen-Schmidt reaction. In cases where the product formed still has a reactive alpha hydrogen and a hydroxide adjacent to an aromatic ring, the reaction will quickly undergo dehydration leading to the condensation product.

Because of the symmetry of acetone the reaction can now be repeated on the other side of the carbonyl leading to the final product, dibenzalacetone, a useful molecule which has been employed as an ultraviolet blocker in sunscreen preparations.
Procedure
Use a micropipet to transfer 6 millimoles of benzaldehyde to a 25x100 mm test tube. Use a micropipet to transfer 3 millimoles of acetone to the benzaldehyde then
dissolve the resulting mixture in 3 mL 95% ethanol by stirring with a glass rod. Add 1 mL 10% NaOH and stir until a precipitate begins to form, then let the mixture stand
with occasional stirring for 20 minutes.
Cool the mixture in an ice bath for 5-10 minutes, then transfer the liquid to a 30 mL beaker. Rinse the reaction tube with about one-half mL ethanol to transfer any remaining crystals from the test tube into the beaker. Use a pipet to remove the liquid from the solid product in the beaker. Add 2 mL ice water to the crude product and stir to wash the crystals, then remove the water with a pipet. Repeat with a second ice water wash. After removing as much water as possible, transfer the solid back to the reaction tube for recrystallization. Rinse the beaker with 1-2 mL ethyl acetate to transfer any remaining crystals from the beaker to the test tube. Recrystallize the solid from a minimum volume of hot ethyl acetate (see Organic Syntheses link for rough quantity), cool to recrystallize and collect the product by vacuum filtration on a Hirsch funnel.
After drying, obtain the mass of the product, percent yield, and melting point.
Prelab Questions
Use online (or other) sources to answer the following questions prior to laboratory.
| 1. Using molecular weight, determine the mass (in milligrams) of 6 millimoles of benzaldeyhde. |
| |
| 2. Using density and mass, determine the volume (in microliters) of 6 millimoles of benzaldehyde. |
| |
| 3. Determine the mass (in milligrams) of 3 millimoles of acetone. |
| |
| 4. Determine the volume (in microliters) of 3 millimoles of acetone. |
| |
| 5. Calculate the theoretical yield of product (in grams). |
| |
| 6. Determine the volume of ethyl acetate used to recrystallize 1 g of product. Use the Organic Syntheses link to answer this question. |
| |
| 7. Based on your theoretical yield, how much ethyl acetate will you need to use to recrystallize your crude product? |
| |
| 8. Find a literature melting point for the product, dibenzalacetone. Include reference to source. |
|
Literature Sources
Organic Syntheses: Synthesis of Dibenzalacetone
The Aldol Reaction
The Claisen Condensation
The Science of Sunscreens
Aldrich Chemical Company Search Page
ChemExper Chemical Directory
Acros Chemicals
NIST Chemistry Webbook