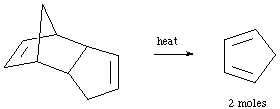
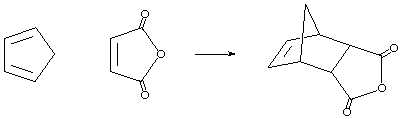
In this laboratory we will perform a cycloaddition reaction between a conjugated diene (cyclopentadiene) and a dienophile (maleic anhydride) to yield an interesting Diels-Alder adduct. The reaction begins by the preparation of cyclopentadiene via the retro Diels-Alder reaction of dicyclopentadiene.

This reaction can be performed by simply boiling the dicyclopentadiene and fractionally distilling the cyclopentadiene as it forms. To prevent it from dimerizing back to dicyclopentadiene we will distill directly into the dienophile solution, thus facilitating the reaction.
Procedure
Dissolve 350 mg maleic anhydride in 1.5 mL ethyl acetate by gently warming in
flat bottomed culture tube. Add 1.5 mL petroleum ether to the solution.
Note: petroleum ether is a mixture of alkanes. It is NOT an ether. You
may subsitute another non-polar solvent such as pentane or hexane.
Place 4.5 mL of dicyclopentadiene and a boiling chip into a 25 mL round bottom flask. Assemble apparatus for the fractional distillation of the dicyclopentadiene, using the test tube from the first step as the distillation receiver. Heating very gently with a heating mantle, begin the distillation of dicyclopentadiene.
Pre-lab questions:
1. Consider the materials used in today's lab. Which two materials represent the greatest safety conerns?
2. Convert 350 mg maleic anhydride to millimoles.
3. What is the boiling point of ethyl acetate?
Watch the condenser carefully. Count the drops of distillate. Distill a total of 35 drops, then stop heating. CAUTION: Be certain that the flask containing the dicyclopentadiene does not boil to dryness!
Pre-lab questions:
1. What is the boiling point of dicyclopentadiene?
2. What is the boiling point of cyclopentadiene?
Turn off the heat. Remove the distillation receiver. Cap the tube and tightly seal with parafilm. Place in a secure spot in your locker and allow the crystals to form slowly. Next week isolate the crystals, allow them to dry, then weigh the product and record melting point and yield.
Pre-lab questions:
1. What is the density of cyclopentadiene?
2. Assuming that 20 drops of cyclopentadiene is about 1 mL, about how many millimoles of cyclopentadiene are we adding to the maleic anhydride?
Resources
Background material
The Diels-Alder reactionChemical Safety Data:
UConn Organic Chemistry Server, Named Reactions
Oxford University, Named Organic Reactions
Nobel Prize in Chemistry, 1950
Online sources for melting point, formula weight, etc.
Chemical calculations needed to complete lab: