Selective Oxidation of Benzyl Alcohol to Benzaldehyde
Adapted
from literature1 by Keti Assor, Irvin Levy, Erin Thames and Rowan
Walker
Background
Traditionally
oxidation of alcohols to aldehydes requires the use of hazardous, heavy-metal
reagents, such as
pyridinium chlorochromate (PCC). In fact, PCC has been widely
considered an appropriate agent for the oxidation, appearing in many
undergraduate textbooks as the only viable method to produce aldehydes from
alcohols. Both PCC and the usual solvent for its reaction, dichloromethane, are
hazardous, cancer suspect agents. In this particular reaction, an
environmentally safer oxidizing agent is formed from sodium molybdate to make
an efficient catalyst that is activated by aqueous hydrogen peroxide. In addition,
hydrogen peroxide serves not only as an activating agent, but also as the
solvent for this transformation.
Purpose
This
experiment provides an excellent example of selective oxidation. In addition it
demonstrates the synthesis and use of a catalyst in reaction. Other green
lessons include replacement of a hazardous reactant with a safer alternative,
the elimination of hazardous solvents, and reduction of waste.
Procedure
Preparation of tetrakis(benzyltriethylammonium)
octamolybdate catalyst
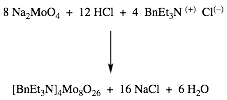
Scheme I:
Method:
To
prepare the catalyst, sodium molybdate dihydrate (0.30 g; 1.2 mmol), and 4 M
HCl (0.5 mL; 2.0 mmol) were added to a vial. About 1 mL of water was added to
complete dissolution. Into a second vial benzyl tri-ethyl ammonium chloride (BTEAC)
(0.525 g; 2.30 mmol) and ca. 3 mL
water were stirred until dissolved. The BTEAC solution was heated at 70 °C with
stirring. To the stirred solution was added the molybdate solution dropwise.
After addition was complete, the solution was stirred for an additional five
minutes, then removed from heat and vacuum filtered. The solid was washed with ca. 5 mL while on the filter. The
catalyst produced could be used wet or saved for later use.
Preparation
of Benzaldehyde
Scheme
II:
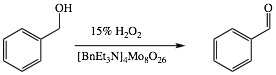
Method:
For
preparation of benzyaldehyde, benzyl alcohol (5 mL; 50 mmol) was added to a round
bottom flask containing octamolybdate catalyst (0.25 g dry weight; 0.2 mol%).
Next, hydrogen peroxide (60 mmol; 12 mL 15% or 60 mL 3%) was added to the flask. The
mixture was then refluxed for one hour, then the
apparatus was modified for simple distillation with glass centrifuge tubes as
receivers, yielding benzaldehyde and water in the distillate. After collecting
about 8 - 10 ml of distillate, the tubes were centrifuged. Water was removed with a
disposable pipet and the product was dried over sodium sulfate, weighed and IR
spectrum was recorded.
Prelab
1. Look
up information, hazards, and safety on all materials used in this procedure.
2.
Look up hazard information for PCC (pyridinium chlorochromate) and
dichloromethane.
3.
The original published procedure calls for the use of 15% hydrogen peroxide.
Look up hazard information for this reagent and compare to the 3% solution. Which do you recommend that we use?
4.
What makes the benzaldehyde oxidation
reaction selective?
5.
What is the atom economy of each
reaction scheme?
6.
What is the approximate e-factor of
each method?
References
1 Guo, Ming-Lin; Li, Hui-Zhen Li. Selective oxidation of
benzyl alcohol to benzaldehyde
with
hydrogen peroxide over tetraalkylpyridinium octamolybdate catalysts. Green Chem.
2007, 9, 421-423.
2 ACS. Selection from “Introduction to green
chemistry”. 2002. Web
access:
http://domin.dom.edu/faculty/jbfriesen/chem254lab/atom_economy.pdf
3 Anastas, Paul T.; Warner, John C. Green Chemistry: Theory and Practice;
Oxford University Press: Oxford,
1998.
4
Levy, Irvin J. The goal is zero; E-factor as a green
chemistry metric.
http://www.cs.gordon.edu/~ijl/visualizingWaste/