
While synthesis is fundamental to organic chemistry, this laboratory is not primarily concerned with the formation of the product. Indeed, this particular reaction is not studied in detail until midway through the second semester in our course. Of greater interest is the purification of our products after the actual reaction is complete. Organic chemists refer to this purification process as "workup" for the synthesis. In this lab we are primarily interested in developing techniques for workup when the product is a solid. Our workup will involve recrystallization, isolation via vacuum filtration, and evaporation.
Recrystallization is a process by which crude (impure) crystals are purified by dissolving in the smallest possible volume of a hot solvent. The solvent must be chosen carefully. The main requirement is that the crystals are much more soluble when the solvent is hot than when it is cold. The saturated solution is then cooled and the purified product solidifies. These crystals are then filtered and set aside to dry.
The filtrate obtained from a recrystallization is referred to as the mother liquor of the crystals. Since some of the product will remain dissolved in the mother liquor, it is often desirable to evaporate a large portion of the solvent, yielding a concentrated filtrate which can be cooled yielding a second crop of crystals.
Note that the procedure below is designed with the environment in mind. For example, we are using very small amounts of material. This allows the work to proceed rapidly, safely, inexpensively and with small amounts of by-products. The small scale of this approach also forces one to spend extra effort on careful technique.
Further the reaction is unusually friendly to the environment in that no solvent is required to cause the reaction to proceed (consequently there will be much less waste in the final process) and the recrystallization solvent is environmentally benign compared to many other solvents which have been used widely in organic chemistry in the past. This conscious effort to perform interesting chemistry while being explicitly mindful of the environmental impact of the process is referred to as practicing "green chemistry." We will talk more about this concept as the course proceeds.
Procedure
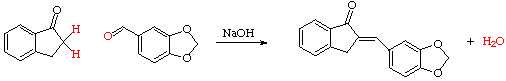
Place 250 mg of piperonal and 200 mg of 1-indanone into the flat-bottom culture
tube in your glassware drawer.
Taking care not to break the tube, use a metal spatula to mix the materials
thoroughly for about 1 minute. Note any observations that present themselves.
After the material has liquefied, add 50 mg powdered sodium hydroxide (CAUTION:
avoid contact!). Note: sodium hydroxide is very hygroscopic. PLEASE keep the
top on the bottle and try to work in such a way as to minimize exposure of the
powdered sodium hydroxide to the moisture in the room air.
Stir the sodium hydroxide into the mixture for about 1 minute, then set aside for 15 minutes to allow the reaction to go to completion. Add 2 mL of 10% hydrochloric acid, scraping and stirring to be sure that the solid product is thoroughly mixed with the HCl. Using a drop of liquid from the tip of the spatula, test the solution with litmus paper to be certain that it has become acidic.
At this point the synthesis is complete; now the workup begins. Decant the liquid from the tube into a small waste beaker. We will now recrystallize the crude product in the culture tube. To the tube, add about 20 mL 90% ethanol/water solvent and a boiling chip. Warm over a hot water bath until all of the product dissolves, stirring while warming to aid the solution process. It will be convenient to share a water bath with another student. It might be necessary to add an additional 5-10 mL of ethanol to obtain complete solution, but add in several small portions, seeking to dissolve the product in the smallest possible amount of boiling solvent.
Once the product has dissolved, remove from the hot water, cap the tube and allow the solution to cool by placing the tube into an ice water bath. Do not allow water from the ice bath into the tube. After cooling 5-10 minutes the main crop of purified crystals may be isolated on the small Büchner funnel by vacuum filtration. Place the wet filter paper onto a watch glass. Label the watch glass, "2-(piperonylidene)indan-1-one, 1st crop" and allow the crystals to dry until the next laboratory period.
Transfer the mother liquor back to the culture tube, add a boiling chip and heat over hot water until the total volume of liquid is reduced to about 1/3 its original volume. Place a vacuum hose in the mouth of the tube to hasten the evaporation process. After evaporation is complete, cap the tube and cool in the ice bath to induce the formation of a second set of crystals. (Note: if necessary, this is a good stopping point. Rather than cooling in the ice bath, simply cap the tube, wrap with parafilm, label, and leave in the locker; crystals should form before the next laboratory session). Collect these crystals in the same way and place them on a second watch glass. Label the watch glass, "2-(piperonylidene)indan-1-one, 2nd crop" and allow the crystals to dry until the next laboratory period. Dispose the waste liquid into the organic waste container and make a notation on the waste log of the material placed in the container.
Observe the appearance of both crops of product. Note color, shape, and any other details which you can observe. Determine the mass of the two crops. Determine the purity of the crops by carefully measuring the melting point of each in duplicate. Comment on the relationship between appearance and apparent purity of the samples.
Your notebook conclusions should include a determination of limiting reagent in this synthesis, a calculation of your percentage yield (based on the mass of the combined crops of crystals), tables of the melting points obtained for all samples and any relevant comments.
Prelab Questions
References
Adapted from Green Organic Chemistry, Strategies, Tools, and Laboratory Experiments
by Kenneth M. Doxsee and James E. Hutchison, University of Oregon.
G. Rothenberg, A. P. Downie, C. L. Raston and J. L. Scott, "Understanding Solid/Solid Organic Reactions,"
J. Am. Chem. Soc. 2001, 123, 8701-8708.
Background on green chemistry:
Chemical calculations needed to complete lab:
Online sources for melting point, formula weight, etc.