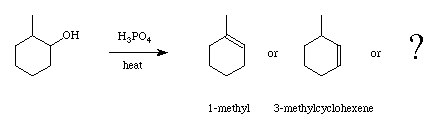
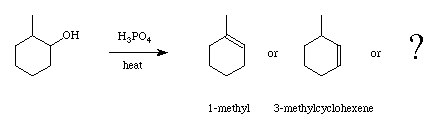
Careful consideration of the mechanism should give clues as to the identity of another product. After forming the product we will use gas chromatography to analyze the composition. In this laboratory we will perform a synthesis and a workup in the same step. This is possible because the reagents used to synthesize methylcyclohexene by alcohol dehydration all have higher boiling points than the product. Since the reaction is run at high temperature it is easy to perform a fractional distillation to remove the product as it forms. This also helps improve the yield in the reaction by shifting the equilibrium toward the formation of product (Le Chatelier's rule).
Procedure
To an 13x100 mm test tube, add 4 mL of water then use a piece of label tape to mark the volume contained in the tube. Empty the tube and set aside. To a 25 mL round bottom flask, add 6 mL of 2-methylcyclohexanol, 5 mL of 85% phosphoric acid and a boiling stone. Set the flask up for fractional distillation using the 18x150 mm test tube as the receiver. Slowly heat the flask to boiling using a sand bath as the heat source. Continue heating at such as rate as to keep the temperature of the distillate vapor below 100°C. Continue until you have collected 4 mL of product, then lift the apparatus from the sand bath to cool.
Use a dropper to remove a layer or bubble of water which may be present at the bottom of the test tube. Dry over a suitable drying agent (e.g. Na2SO4 or CaCl2). After 5-10 minutes, filter the product through a cotton plug in the stemless funnel, collecting the liquid in a vial. Immediately cap the vial.
Perform a GC analysis of the product. It is possible to distinguish the 1-methyl peak from the 3-methyl peak by running a second chromatogram. The sample for the second chromatogram is prepared by mixing 2 drops of product with 2 drops of known 1-methylcyclohexene. In this chromatogram the 1-methyl peak will be much larger than in the previous chromatogram. This is referred to as "spiking" the sample.
Calculate the percentage yield of methylcyclohexenes. To do this you will need to look up the density of 2-methylcylohexanol, 1-methyl and 3-methylcyclohexene so that you can convert mL used or collected into grams and then moles.
Pre-lab questions
Assume that 6 mL of 2-methylcyclohexanol is completely converted to 1-methylcyclohexene. Use online reference data for molecular weights, densities, etc. to answer the following questions.
| 1. Use density to calculate the mass of 6 mL of 2-methylcyclohexanol. |
| |
| 2. Use molecular weight to convert the previous answer to moles. |
| |
| 3. Assuming that the alcohol is completely converted into 1-methylcyclohexene, how many moles of 1-methylcyclohexene could theoretically be formed? |
| |
| 4. Convert the previous answer from moles to grams. |
| |
| 5. Convert the previous answer from grams to milliliters. |
| |
| 6. Assuming that we collect 4 mL of the product, what is our yield? |
|
Literature Sources
Elimiation reaction mechanisms
Aldrich Chemical Company Search Page
ChemExper Chemical Directory
Acros Chemicals
NIST Chemistry Webbook