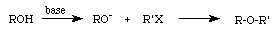
In this laboratory we will utilize the nucleophilic substitution reaction to prepare an ether. This method, referred to as the Williamson ether synthesis, follows a straightforward general approach:
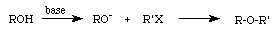
In specific, we will begin with 4-methylphenol as our initial alcohol. Phenols are unusually acidic alcohols; consequently, we can use sodium hydroxide to convert a phenol to its conjugate base rather than a more powerful reagent. We will use chloroacetic acid in the "alkyl halide" step of the reaction strategy:

Method
(Warning! Diethyl ether in use. ABSOLUTELY NO FLAMES IN THE LAB!!!)
Accurately weigh about 1gram of 4-methylphenol (p-cresol)
(caution: avoid contact, skin irritant and toxic compound) into a
25x100 mm test tube. Using a graduated pipet, add 5 mL of 30% aqueous
NaOH (caution: avoid contact, may cause severe chemical burns!).
Next add 1.5 g of chloroacetic acid (caution: avoid contact, skin
irritant!) to the test tube. Stir to dissolve the reagents -- gentle
warming may help as may the addition of water (added dropwise with
swirling).
Clamp the test tube in a hot water bath (90-100°C) for 30 to 40 minutes. Cool the tube and dilute the resulting mixture with about 10 mL of water. Add 6M HCl until a drop of liquid on the end of a stirring rod causes blue litmus paper to turn red (i.e. until the solution is acidic).
Transfer the acidic liquid and any solid residue to a small (125 mL) separatory funnel and extract the solution with 15 mL of diethyl ether (caution: avoid inhalation of vapors). If two distinct liquid layers do not result, add 5 mL water and 5 mL of ether and repeat as needed. Drain the aqueous layer into a beaker and set aside for later disposal. Wash the ether layer with about 15 mL of water. After removing the aqueous layer, extract the ether layer with about 10 mL of saturated sodium bicarbonate solution. Note that it is particularly important to vent frequently in this step. It is also important to mix the layers thoroughly or the desired extraction will not occur. If a solid layer appears in the separatory funnel, add water and sodium bicarbonate solution to the funnel (one dropperful at a time) until the funnel contains two distinct layers. Drain the sodium bicarbonate layer into a a small beaker. Cautiously acidify the bicarbonate layer with 6M HCl -- you must add the HCl very slowly at the beginning because extensive foaming will occur. Once the addition of more HCl results in very little noticeable additional foaming, the solid product may be filtered using the Büchner funnel.
Place a small sample of the crude product aside and recrystallize the remaining product from the smallest possible volume of hot water.
Place the purified crystals aside to dry. Determine the mass and melting point of both sets of crystals. Literature melting point = 136-137°C.
(laboratory adapted from the procedures in "Experimental Organic Chemistry" by Durst and Gokel and "Experimental Methods in Organic Chemistry" by Moore, Dalrymple and Rodig.)